Популярные вопросы форума
СпроситьВопросов нет. Будьте первым, кто задаст вопрос!
Документы
Background
The FreeStyle Libre Pro system was developed by Abbott Diabetes Care as part of its advancements in Continuous Glucose Monitoring (CGM) technology. This system utilizes a small sensor worn on the body to continuously measure interstitial fluid glucose levels. The underlying FreeStyle Libre technology was designed to be user-friendly, notably featuring factory calibration, which eliminates the need for patients to perform fingerstick blood glucose checks for system calibration. Initially launched in Europe around 2014, the FreeStyle Libre Pro was the first product using this technology to receive U.S. FDA approval in September 2016, marking the company's official entrance into the American CGM market.
The FreeStyle Libre Pro was strategically introduced as a professional-use, "blinded" system. In this design, the patient cannot view their glucose data in real-time. This blindness allows healthcare providers to obtain an unbiased, comprehensive 14-day record of a patient's glucose patterns and variability. The collected data is then analyzed retrospectively by the provider after the sensor is removed, serving as an objective basis for making informed therapy adjustments. This establishment of the sensor's clinical efficacy and regulatory confidence paved the way for the later approval of the unblinded, personal-use FreeStyle Libre system in 2017, which provides patients with immediate glucose data for daily self-management.
Identification
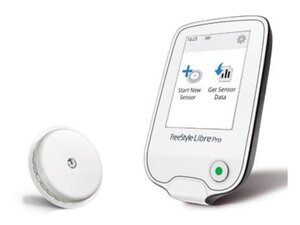
The key identifying feature is that the patient, after the sensor is applied to their arm, is not given a reader or smartphone app access to view their glucose levels. The sensor silently and automatically records a full two weeks of data, which is then downloaded using a separate professional reader device kept at the clinician's office once the patient returns. This setup means the patient is unable to make real-time adjustments to their diabetes management, as the entire purpose is to provide the clinician with a comprehensive, unbiased picture of daily glucose trends for treatment guidance.



Technical Specifications
Sensor:
Wear Time - Up to 14 days
Glucose Measurement - Measures glucose in the interstitial fluid automatically every minute
Data Storage - Stores glucose levels in 15-minute intervals, capturing up to 1340 glucose results for up to 14 days.
Sensor Size - Approximately 0.2 inches x 1.4 inches
Placement - Back of the upper arm (applied by a healthcare professional)
Water Resistance - Water-resistant (can be worn in the shower, while exercising, etc.)
Calibration - No fingerstick calibration required (it is factory-calibrated)
Patient Interaction - None required by the patient after application (it's a "blinded" CGM)
Power Source - 1 silver oxide battery (contained within the disposable sensor)
Reader:
Device Type - Hand-held device for the healthcare professional
Function - Activates the sensor, and later is used to scan and download the 14 days of glucose data stored in the sensor.
Data Download - A single scan takes as little as five seconds to download the data.
Wireless Communication - Uses wireless radiofrequency communication. The sensor and reader must be within about 1.5 inches (4 cm) of each other to scan.
Interface - Typically includes a touchscreen color display and a micro-USB port for charging and data upload.
Alerts/Alarms - None on the system itself, as the data is blinded and reviewed retrospectively by the clinician
Software - Data is analyzed using the LibreView™ FreeStyle Libre Pro Software.
Accuracy - The system has a MARD (Mean Absolute Relative Difference) of 12.3% over 14 days without fingerstick calibration.
The FreeStyle Libre Pro is specifically a professional-use system. The data is blinded (not viewable by the patient) and is intended for the healthcare professional to analyze retrospective trends and patterns to guide therapy decisions. The personal-use versions (like FreeStyle Libre 14 day, 2, or 3) have different specifications and features, such as on-demand scanning or real-time glucose readings/alarms for the user.